Call us:
1234567890Email:
info@argentum-llc.com
Call us:
1234567890Email:
info@argentum-llc.com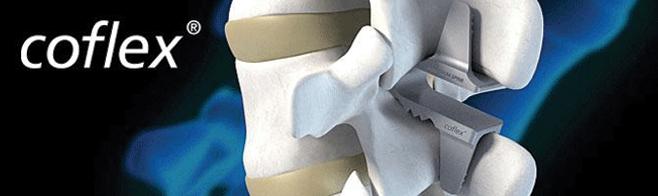
Argentum is proud to carry Paradigm Spine LLC’s COFLEX® line of spinal implants. The COFLEX® Interlaminar Technology is the 1st and only motion preserving minimally invasive treatment for moderate to severe spinal stenosis post decompression.
The coflex device is a U-shaped, titanium alloy implant that fits between two bones called the spinous processes located in the lower back (lumbar region) of the spine. The device is placed between two adjacent lower back bones after surgical relief of pressure on the spinal cord and nerve (decompression) to ease the pain associated with lumbar spinal stenosis, a narrowing of the passages for the spinal cord and nerve.
This devices helps to relieve some or all of the symptoms of lumbar spinal stenosis. Unlike a fusion procedure, the coflex device is designed to allow some movement at the affected level(s). It has In measures such as pain relief, outcomes and patient satisfaction obtained in clinical trials that led to the FDA approval to market, the coflex device demonstrated clear superiority. There were also clear advantages in terms of reduced operative time, reduced blood loss during surgery, reduced hospital length of stay and lesser need for post-operative narcotics.
Click here for more information on COFLEX® Interlaminar Technology.

Argentum is a stocking distributor for STERIPLEX® SD, the only EPA- registered C. difficile Sporicide, Virucide, Tuberculocide, Bactericide and Fungicide that is practical for daily use. STERIPLEX® SD’s demonstrated efficacy is reflected in the reduction of Hospital Acquired Infections (HAIs) during hospital trials and is a primary reason why Infection Control & Prevention Specialists are switching to the disinfectant.
The safety profile of STERIPLEX® SD has been proven through rigorous testing and clearly differentiates it from competing disinfectants that utilize dated technology, are highly toxic and environmentally unfriendly.
STERIPLEX® SD is the only C. difficile sporicide with a Category IV rating for Primary Eye and Skin Irritation, and for Acute Oral, Dermal and Inhalation Toxicity with no Precautionary or First Aid Statements required by the EPA, and is the only C. difficile sporicide with an HMIS healthy and physical hazard rating of zero(0).
STERIPLEX® SD’s demonstrated efficacy is reflected in the reduction of HAIs during hospital trials and is a primary reason why Infection Control & Prevention Specialists are switching to the disinfectant.
STERIPLEX® SD belongs to a family of powerful infection and contamination control products that have significant applications in multiple industries and markets. Formulations based on the STERIPLEX® technology platform, such as the anthrax-fighting product STERIPLEX ULTRA™ were the very first disinfectant sporicides to be registered by the EPA.
Click here for more information on STERIPLEX® SD daily use sporicidal disinfectant.